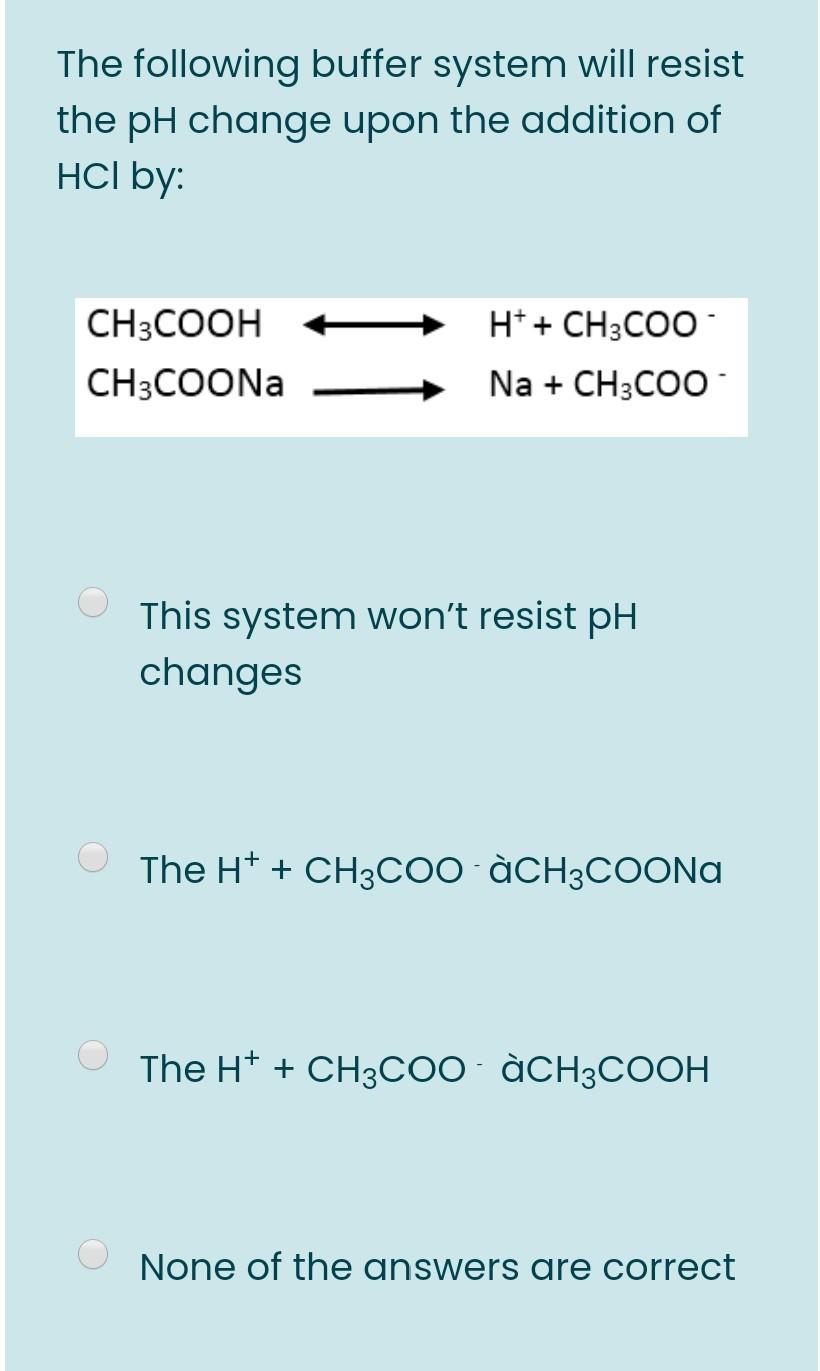
/is2.ecplaza.com/ecplaza2/products/8/8f/8f7/1187082394/sodium-sodium-ethanoate.jpg)
Sodium sodium ethanoate Acetate CH3COO Na NaOAc C2H3NaO2 C2H3NaO2-3H2O - Lianyungang Zhaofu Mineral Co.,Ltd.
44. CH3CCCH3+Pd/CaCO3+(CH3COO)2Pb=B,CH3CCCH3+Na in liq NH3=A,the minimum heat of hydragention in which between A and B?
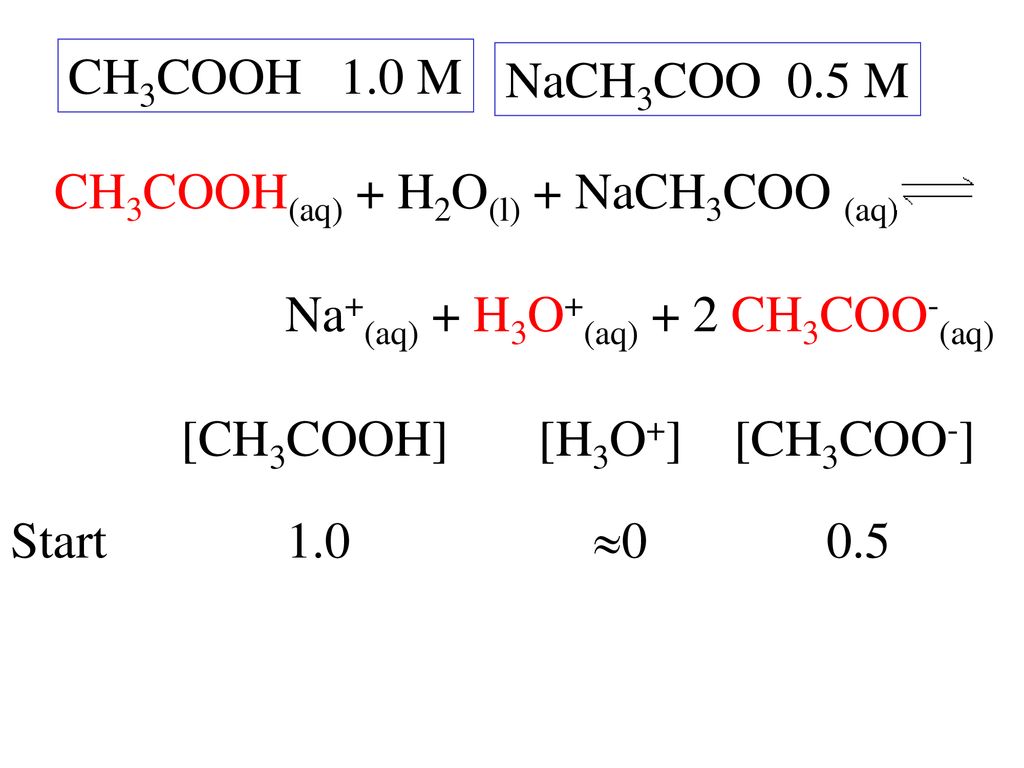
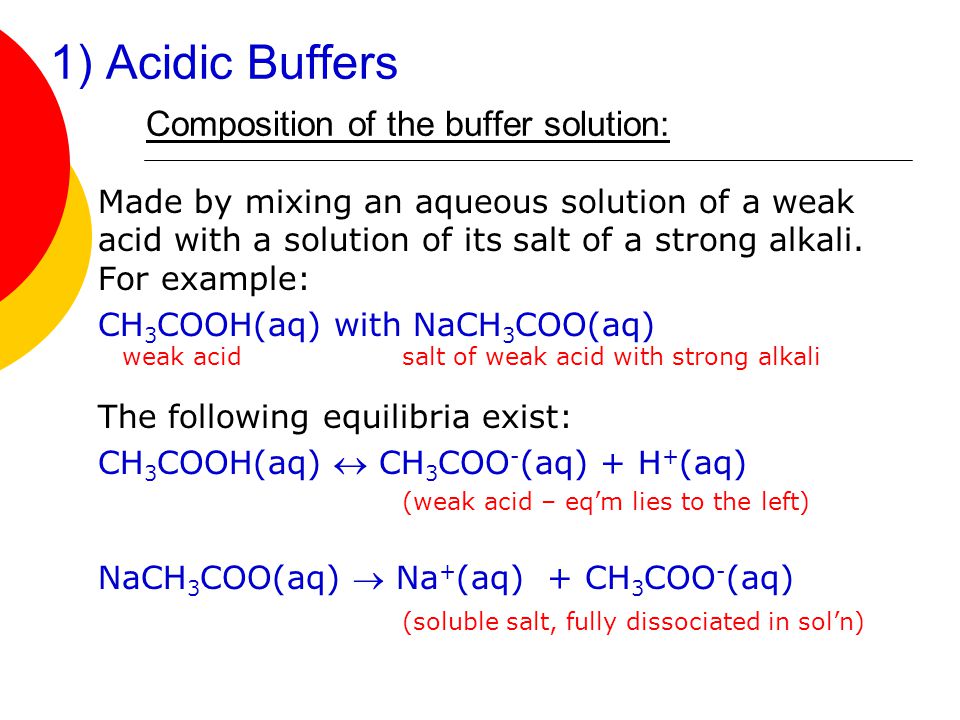
SOLVED: 1.0 mL of 6.0 M HCl is added to 100 mL of a buffer that contains equal moles of acetic acid (CH3COOH, Ka = 1.8 × 10-5) and sodium acetate (Na (CH3COO)).
SOLVED: If 10 mL of 1M NaOH are added to one liter of a buffer that is 0.3 M acetic acid and 0.2 M sodium acetate (Na+CH3COO–), how much does the pH

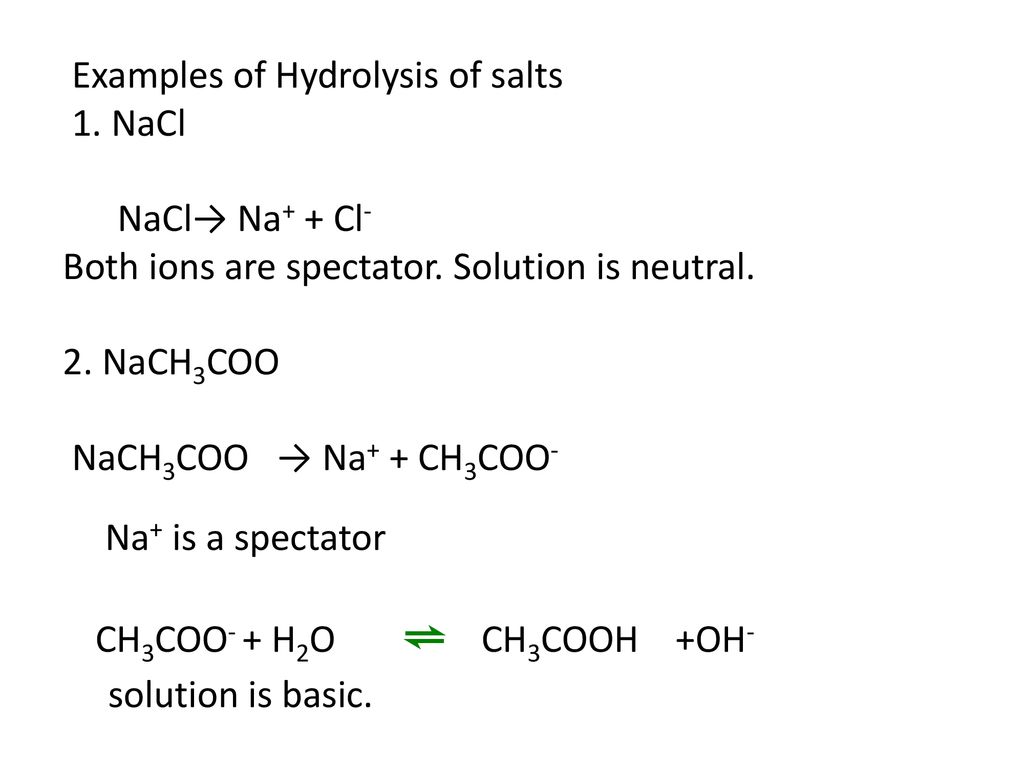
What is the pH of a 0.402 M aqueous solution of NaCH3COO? Ka (CH3COOH) = 1.8x10-5 | Homework.Study.com
Why does the solution of sodium acetate give more concentration of Hydroxide ion? Shouldn't the number of Hydroxide ion and hydrogen ion be equal? - Quora